There was a big announcement yesterday on the Covid-19 cure and predictably Donald Trump and his Vice Poodle have already made attempts to take the credit for the 90% news. Of course in reality they had nothing to do with it.
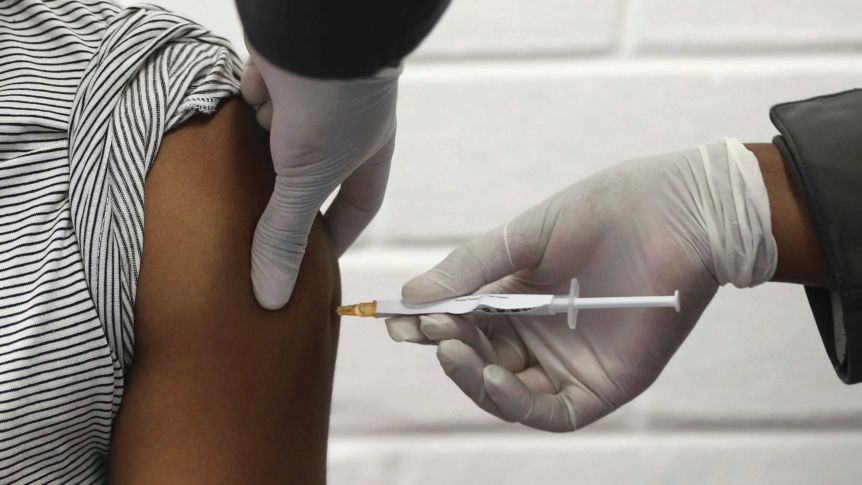
Drug companies Pfizer and BioNTech announced their vaccine candidate was “more than 90 per cent effective in preventing COVID-19” in people who, as far as they know, have not previously been infected with the virus.
The vaccine, dubbed BNT162b2, involves two jabs, each three weeks apart.
In interim results from the companies’ phase 3 clinical trial, which started in July and has more than 43,000 enrolled participants, they reported 94 lab-confirmed COVID-19 infections.
So to a world still on life support it sounds pretty good, hey?
But the vaccine‘s ‘efficacy’ doesn’t measure how well it stops the SARS-CoV-2 virus entering a vaccinated person’s body.
Instead, it’s a measure of stopping — or at least reducing the severity of — COVID-19.
And this vaccine, apparently, lowers your chances of getting sick by 90 per cent, compared to someone who hasn’t been vaccinated.
Kylie Quinn, a vaccine expert at RMIT University, told ABC News: “If you had 10 people who you knew were going to be infected … and you vaccinated those people before they were exposed, nine out of those 10 people would not develop (COVID-19).”
If you want to get technical (and who doesn’t?!), vaccine efficacy and vaccine effectiveness are slightly different terms.

Vaccine efficacy is calculated through clinical trials, like the Pfizer/BioNTech trial. Vaccine effectiveness is measured out in the real world, once the vaccine has been approved for use in the general population.
To calculate the more-than-90-per-cent efficacy figure for BNT162b2, an external committee examined how many of the 94 infected individuals were vaccinated and how many received the placebo (a saline injection).
But the published results are a little light on detail. The announcement was made via press release — not a peer-reviewed journal paper — and it did not include the vaccine/placebo breakdown of infected participants.
The efficacy of the vaccine may change over time, too.
Dr Quinn says there’s still a little way to go before the trial wraps up.
There are currently 11 COVID-19 vaccines in phase 3 clinical trials, but Pfizer is the first company to announce its results.
While Pfizer is still collecting safety data on the vaccine, it says so far no serious safety concerns have been observed. It plans to apply for emergency authorisation from the US Food and Drug Administration in late November.
Clinical trial stages
- Pre-clinical: Testing in animals. Does the vaccine produce antibodies? Does it protect against illness? What dose is necessary?
- Phase I: Testing in a small number of humans. This phase is about making sure the vaccine is safe.
- Phase II: More testing in humans — does the vaccine actually work?
- Phase III: Testing in a larger number of humans to confirm its effectiveness.
- Phase IV: After the vaccine has been rolled out, ongoing surveillance to make sure it’s safe and doesn’t have long-term adverse effects.
The early trial results aren’t just good news for Pfizer either, according to Dr Richard Hatchett, CEO of the Coalition for Epidemic Preparedness Innovations (CEPI).
“We believe these interim results also increase the probability of success of other COVID-19 candidate vaccines which use a similar approach,” Dr Hatchett said in a statement.
Whilst it is still no silver bullet, it does sound promising.
Subscribe to FIB’s Weekly Alchemy Report for your weekly dose of music, fashion and pop culture news!







